Background: Chronic anemia contributes to morbidity and mortality in sickle cell disease (SCD) and is associated with stroke, leg ulcers, pulmonary hypertension (PH), priapism, and chronic kidney disease (CKD). Treatment options for anemia in SCD are limited - hydroxyurea (HU) is the gold standard treatment, but hemoglobin (Hb) responses to HU in adult and pediatric cohorts appear to be modest at best. We evaluated the Hb response to HU and voxelotor in an adult SCD cohort, as well as baseline factors associated with Hb response to HU.
Methods: This longitudinal study was based on retrospective chart review of patients with SCD followed in the UPMC Adult Sickle Cell Program, with drug start dates between 2007 and May 2023. Subjects with a documented start date for HU/voxelotor, a baseline Hb ≤ 3 months prior to starting drug, and a follow-up Hb at 1 or 3 months (± 2 weeks) after the start date were included. Those who self-discontinued therapy were excluded. Baseline factors were collected within ≤ 3 months of drug start date. Given the small number of subjects on voxelotor, factors associated with Hb response were evaluated in the HU group only. Baseline factors were reported using descriptive statistics and compared between Hb responders (defined as Hb increase ≥ 1 g/dL from baseline - a common outcome measure in clinical trials of anti-polymerization agents) and non-responders. Linear regression analysis was performed with change in Hb at 1 month (ΔHb) as the outcome of interest, as Hb levels at 1 and 3 months were highly correlated (r=0.905, p<0.001) and there were fewer missing values for Hb at 1 month. Variables associated with ΔHb with p<0.25 were included in a multivariable regression model. P<0.05 was considered significant, and 95% confidence intervals (CI) were reported.
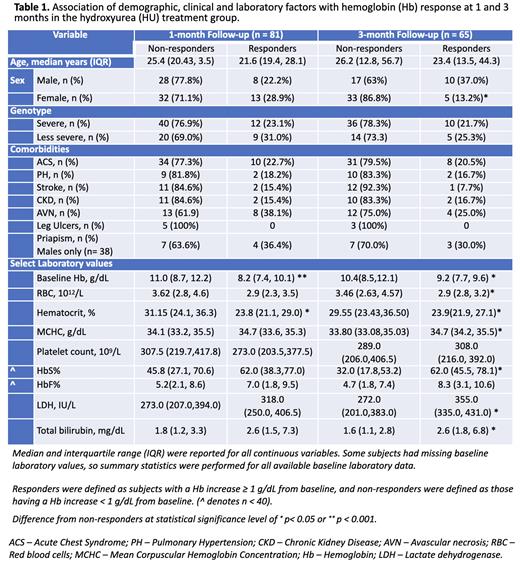
Results: Out of 394 subjects, 93 met eligibility criteria, including 88 starting HU and 5 starting voxelotor. One subject in the voxelotor group was on stable-dose HU. Median ages at start of HU and voxelotor were 24.5 years (range 13-57) and 45.4 years (range 22-60), respectively. Females accounted for 50/88 (56.8%) HU subjects and 4/5 (80%) voxelotor subjects. HbSS genotype made up 47/88 (53.4%) of the HU group and 4/5 (80%) of the voxelotor group. Median baseline Hb in the HU group was 9.8 g/dL, with a median increase of 0.2 g/dL and 0.3 g/dL at 1 and 3 months, respectively. In contrast, baseline Hb in the voxelotor group was 7.2 g/dL with a median increase of 1.9 g/dL and 2.1 g/dL at 1 and 3 months, respectively. Hb response was observed in 21/88 (24%) subjects in the HU group vs. 4/5 (80%) subjects in the voxelotor group. Among responders, median Hb increase was 2.2 g/dL (1 month) and 2.0 g/dL (3 months) with HU. Median baseline ferritin was 200 ng/mL in the HU group and 2413 ng/mL in the voxelotor group.
In the HU group, lower baseline Hb was associated with female sex, severe SCD genotype (HbSS, HbSβ 0), and higher HbS%, WBC count, platelet count, and hemolytic markers (lactate dehydrogenase, total bilirubin, and reticulocyte count) (data not shown). Demographic/clinical variables were not associated with Hb response, except for male gender at 3 months (Table 1). Lower baseline Hb, RBC count, and hematocrit and higher MCHC, HbS%, LDH, and total bilirubin were associated with positive Hb response at 3 months. HbF% was higher in the responder groups but was not significant. In a multivariable linear regression model including age, PH, leg ulcers, CKD, baseline Hb, platelets, and total bilirubin as explanatory variables, baseline Hb was negatively associated with ΔHb (B = -0.26, CI = -0.40 to -0.12, p<0.001). History of leg ulcers was also negatively associated with ΔHb but was not significant (B = -0.94, CI = -2.01 to 0.13, p=0.083).
Conclusion: Overall, Hb response to HU was much lower compared to voxelotor, though in the 24% of subjects who responded to HU, the Hb increase was comparable. Though limited by small sample size, our data revealed that patients started on voxelotor vs. HU were older, more anemic, and more iron overloaded. Subjects with lower baseline Hb had a more robust Hb response to HU. While limited by the absence of dosing and adherence data, this study provides proof of concept of using baseline characteristics to predict Hb response to SCD therapies, demonstrating the feasibility of a personalized treatment approach in SCD. In addition, our data provide real-world evidence of the effectiveness of HU vs. voxelotor for chronic anemia in SCD.
Disclosures
Decastro:GlycoMimetics: Other: Advisory board; Global Blood Therapeutics: Other: Advisory board; Novartis: Other: Advisory board; Forma Therapeutics: Other: Advisory board. Novelli:Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees. Xu:GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios Pharmaceuticals, Inc.: Other: US national principal investigator for the Phase 1 clinical trial pf AG-946 in patients with sickle cell disease..